In kinetic theory we approached temperature as a measure of the concentration of particle-level kinetic energy in a substance. Concentration is a somewhat difficult idea here. Think about how many ways a particle has to move, translational, vibrational, and rotational, and think about how much energy there is on average in each one. That is what temperature measures, and because not all substances have the same number of ways to move at the particle level, substances may have different molar heat capacities. Okay, so that is the kinetic theory approach to temperature which we have been using very fruitfully thus far.
Now that we are moving into the second law of thermodynamics, let us expand our conception of the temperature. Let us begin to look at temperature within a different conceptual framework and start thinking of temperature as a sort of potential function for the escaping tendency of heat.
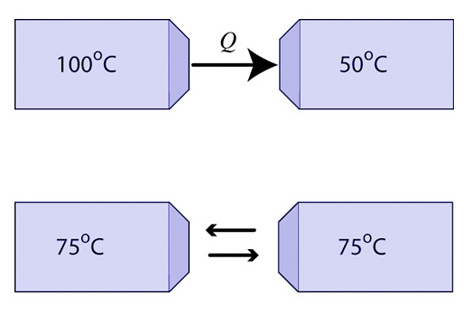
If the temperature is high in a region or area, it will probably not be so for very long. As heat flows from the warmer to the cooler object in thermal contact, the increase in entropy in the cold object is greater than the decrease in entropy for the warm object. Think about that! The heat brings more disorder to the cold object than is lost by the hot object. The total entropy in the universe increases. The temperature difference predicts the direction of spontaneous heat flow.
Let's look at this with a little math. When heat flows from the warm object, it loses an amount of entropy equal to Q/Th (This is a simplification because temperature is changing),and the cold object gains an amount of entropy equal to Q/Tc. Th is greater than Tc, so Q/Th must be less than Tc until the two objects are in equilibrium at the same temperature. Therefore, heat flowing from a hot object to a cold one increases the disorder of the universe. The entropy gained by the cold object is greater than the entropy lost by the warm object and so the heat flow is spontaneous.
Now that we are moving into the second law of thermodynamics, let us expand our conception of the temperature. Let us begin to look at temperature within a different conceptual framework and start thinking of temperature as a sort of potential function for the escaping tendency of heat.
If the temperature is high in a region or area, it will probably not be so for very long. As heat flows from the warmer to the cooler object in thermal contact, the increase in entropy in the cold object is greater than the decrease in entropy for the warm object. Think about that! The heat brings more disorder to the cold object than is lost by the hot object. The total entropy in the universe increases. The temperature difference predicts the direction of spontaneous heat flow.
Let's look at this with a little math. When heat flows from the warm object, it loses an amount of entropy equal to Q/Th (This is a simplification because temperature is changing),and the cold object gains an amount of entropy equal to Q/Tc. Th is greater than Tc, so Q/Th must be less than Tc until the two objects are in equilibrium at the same temperature. Therefore, heat flowing from a hot object to a cold one increases the disorder of the universe. The entropy gained by the cold object is greater than the entropy lost by the warm object and so the heat flow is spontaneous.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |