Matter is made up of minuscule particles which, governed by the thermodynamic conditions, will interact to form one of several possible macroscopic phases: solid, liquid or gas. Although technically, the phases of matter may also include such variations as plasmas and Bose-Einstein condensates among others, in general chemistry our discussion will be concerned with the three phases common on the earth. Solids have a fixed volume and shape. Liquids have a fixed volume but take the shape of the portion of the container they occupy, and gases assume the shape and volume of the container they occupy. Our concern will be with the intrinsic properties of each phase and the thermodynamic factors underlying the transformation of one state of matter to another in phase change processes.
Phase equilibria diagrams, the behavior of real gases, and the vapor pressure related phenomena are perenial MCAT favorites. With liquids, surface tension and capillary action have both shown up on many exams. Occasionally, an MCAT will expect students at least to be familiar with the terminology of crystal structure in solids. In other words, as core general chemistry knowledge, you should expect to see questions from this material on your exam.
WikiPremed Resources
States of Matter Practice Items
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for The States of Matter
A state of matter is one of the many ways that matter can interact with itself to form a macroscopic, homogenous phase.
A solid object is in the states of matter characterized by resistance to deformation and changes of volume.
A liquid is a fluid that can freely form a distinct surface at the boundaries of its bulk material.
The boiling point of a substance is the maximum temperature at which a liquid can remain a liquid at a given pressure.
The enthalpy of vaporization is the energy required to transform a given quantity of a substance into a gas, measured at the boiling point of the substance.
The enthalpy of fusion is the amount of thermal energy which must be absorbed or evolved at the melting point for 1 mole of a substance to change states from a solid to a liquid or vice versa.
The melting point of a crystalline solid is the temperature range at which it changes state from solid to liquid.
The dew point is the temperature to which a given parcel of air must be cooled, at constant barometric pressure, for water vapor to condense into water.
Surface tension is an effect within the surface layer of a liquid that causes that layer to behave as an elastic sheet.
Crystalline solids are a class of solids that have regular or nearly-regular structures, meaning that the atoms in these solids are arranged in an orderly manner
Freezing is the process whereby a liquid turns to a solid.
Evaporation is the process by which molecules in a liquid state spontaneously become gaseous without being heated to boiling point.
Vapor pressure is the pressure of a gaseous phase in equilibrium with its non-gaseous phases.
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume.
Condensation is the change in matter of a substance to a denser phase, such as a gas to a liquid.
An amorphous solid is a solid in which there is no long-range order of the positions of the atoms.
Capillary action is the ability of a substance to draw another substance into it.
A meniscus is a curve in the surface of a liquid and is produced in response to the surface of the container or another object.
A plasma is typically an ionized gas, considered to be a distinct state of matter, apart from gases, because of its unique properties.
A phase is a set of states of a macroscopic physical system that have relatively uniform chemical composition and physical properties
Vapor is the gas phase component present along with a solid or liquid sample of matter which does not completely fill its container.
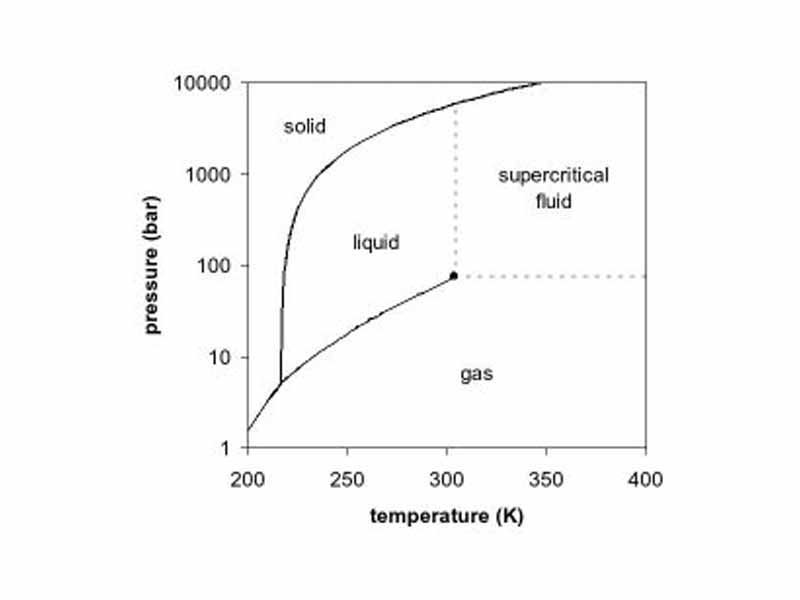
A phase diagram is a type of graph used to show the equilibrium conditions between the thermodynamically-distinct phases.
The triple point of a substance is the temperature and pressure at which three phases (gas, liquid, and solid) of that substance may coexist in thermodynamic equilibrium.
A crystal structure is composed of a motif, a set of atoms arranged in a particular way, and a lattice.
A critical point, also called a critical state, specifies the conditions (temperature, pressure) at which the liquid state of the matter ceases to exist.
A supercritical fluid is any substance at a temperature and pressure above its thermodynamic critical point.
Nucleation is the onset of a phase transition in a small region such as with the formation of a bubble or of a crystal from a liquid.
A cooling curve is a line graph that represents the change of phase of matter, typically from either a gas to a solid or from a liquid to a solid.
The saturation vapor pressure is the static pressure of a vapor when the vapor phase of some material is in equilibrium with the liquid phase of that same material.
The lattice constant refers to the constant distance between unit cells in a crystal structure.
Close-packing of spheres is the arranging of a lattice of spheres so that they take up the greatest possible fraction of a 3-dimensional space.
Vapor-liquid equilibrium is a condition or state where the rate of evaporation equals the rate of condensation.
Superheating (sometimes referred to as boiling retardation, or boiling delay) is the phenomenon in which a liquid is heated to a temperature higher than its standard boiling point, without actually boiling.
Supercooling is the process of chilling a liquid below its freezing point, without it becoming solid.
A ceramic is an inorganic non-metallic materials whose formation is due to the action of heat.
The space group of a crystal is a mathematical description of the symmetry inherent in the crystalline structure.
An aerogel is a low-density solid-state material derived from gel in which the liquid component of the gel has been replaced with gas.
The cubic crystal system is a crystal system where the unit cell is in the shape of a cube.
A crystal system is a category of space groups, which characterize symmetry of structures in three dimensions with translational symmetry in three directions, having a discrete class of point groups.
The Kapustinskii equation calculates the Lattice Energy for an ionic crystal in cases where it is experimentally difficult to determine.
In geometry and crystallography, a Bravais lattice is an infinite set of points generated by a set of discrete translation operations.
The Clausius-Clapeyron relation is a way of characterizing the phase transition between two states of matter, such as solid and liquid, giving the slope of the coexistance curve separating the two phases on a pressure-temperature diagram.
The atomic packing factor or packing fraction is the fraction of volume in a crystal structure that is occupied by atoms.
The Kepler conjecture is a conjecture about sphere packing in three-dimensional Euclidean space which says that no arrangement of equal spheres filling space has a greater average density than that of the cubic close packing and hexagonal close packing arrangements.
The Leidenfrost effect is a phenomenon in which a liquid, in near contact with a mass significantly hotter than its boiling point, produces an insulating vapor layer which keeps that liquid from boiling rapidly.
The term epitaxy describes an ordered crystalline growth on a monocrystalline substrate.
Orthorhombic lattices result from stretching a cubic lattice along two of its lattice vectors by two different factors, resulting in a rectangular prism with a rectangular base.
In the rhombohedral crystal system, the crystal is described by vectors of equal length, of which all three are not mutually orthogonal.
The Schoenflies notation is one of two conventions commonly used to describe crystallographic point groups. The other convention is the Hermann-Mauguin notation, also known as the International notation.
Hermann-Mauguin notation is used to represent the symmetry elements in point groups, plane groups and space groups. This notation is sometimes called international notation.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |