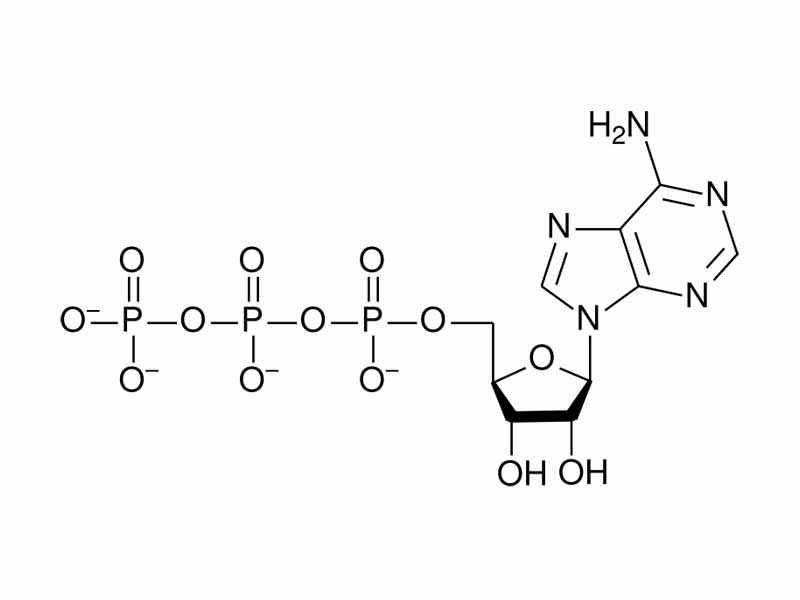
Let us take a specific example of an important biological molecule for metabolism to discuss internal energy decrease in chemistry, ATP. With ATP hydrolysis, internal energy decrease corresponds to the separation of like charge.
ATP contains a series of three negatively charged phosphate groups. When hydrolysis of ATP occurs, one of the phosphate groups, bearing two negative charges, is allowed to separate from ADP, containing the other two negative charges. Just as work would be needed to push negative charges together, potential energy decreases when they are separated. (It is not so simple, though, there is also an additional internal energy decrease because of increased resonance stabilization in ADP, solvent effects, as well as the crucial effects of coordination with magnesium ions. It's not so simple!)
In summary, defamiliarize your perceptions. Think about ATP as a system of charges in molecular form. The three negatively charged phosphate groups are like a coiled spring. The electrostatic potential energy of like charges are held together in ATP, kinetically but not thermodynamically stable. Imagine pushing it all together. You are imagining the high internal energy and enthalpy of ATP. Combine this idea with a sense of the probability of the phosphate being available, just randomly showing up to make ATP from ADP, and you are getting a sense of the free energy. Phosphoryl transfer potential is the 'force that drive the green fuse through the flower' in the immortal words of the poet Dylan Thomas.
ATP contains a series of three negatively charged phosphate groups. When hydrolysis of ATP occurs, one of the phosphate groups, bearing two negative charges, is allowed to separate from ADP, containing the other two negative charges. Just as work would be needed to push negative charges together, potential energy decreases when they are separated. (It is not so simple, though, there is also an additional internal energy decrease because of increased resonance stabilization in ADP, solvent effects, as well as the crucial effects of coordination with magnesium ions. It's not so simple!)
In summary, defamiliarize your perceptions. Think about ATP as a system of charges in molecular form. The three negatively charged phosphate groups are like a coiled spring. The electrostatic potential energy of like charges are held together in ATP, kinetically but not thermodynamically stable. Imagine pushing it all together. You are imagining the high internal energy and enthalpy of ATP. Combine this idea with a sense of the probability of the phosphate being available, just randomly showing up to make ATP from ADP, and you are getting a sense of the free energy. Phosphoryl transfer potential is the 'force that drive the green fuse through the flower' in the immortal words of the poet Dylan Thomas.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |