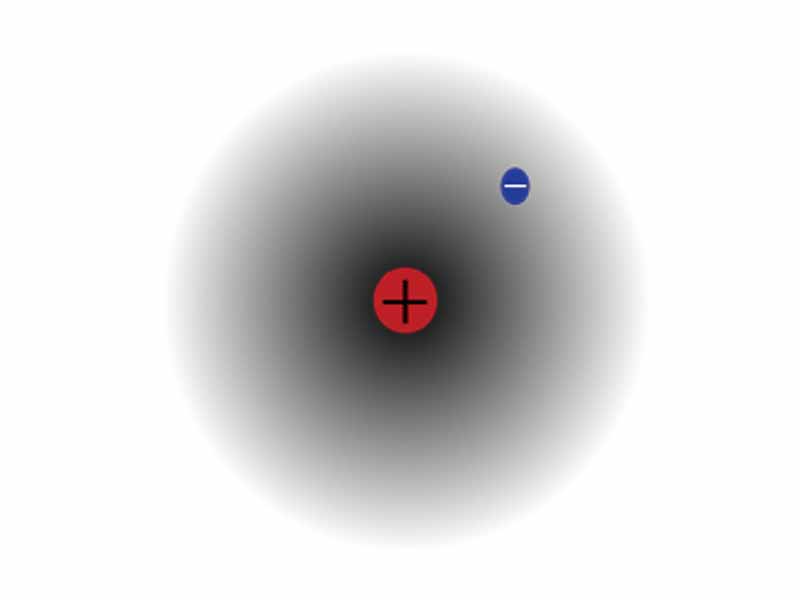
One of our consistent themes in this MCAT course is how helpful classical electrodynamics can be to understanding chemistry. While quantum electrodynamics provides a better model in many ways, plain old Coulomb's Law does provide useful insight into the periodic properties of atomic radius, ionization energy, and electron affinity. Without trying to be perfectly rigorous, imagine mechanical work against electrostatic force to visualize the energy transformations of the atom represented by each periodic property.
This is fine as long as one does not forget that the events are quantized. Atomic radius decreases as you move rightward on the table. Imagine the stronger, more positive nucleus, pulling the electrons tighter with a stronger electrostatic force. Ionization energy increases moving rightward. Imagine how it would take more work to pull an electron in a given shell away from a nucleus with more protons. You are using imaginative visualization to build your intuition.
The classical picture can help you as long as you know its limitations. Sometimes it is helpful to think about the periodic properties as if they were describing a simple electrostatic system as long as you don't forget that quantum electrodynamics provides a fuller picture.
This is fine as long as one does not forget that the events are quantized. Atomic radius decreases as you move rightward on the table. Imagine the stronger, more positive nucleus, pulling the electrons tighter with a stronger electrostatic force. Ionization energy increases moving rightward. Imagine how it would take more work to pull an electron in a given shell away from a nucleus with more protons. You are using imaginative visualization to build your intuition.
The classical picture can help you as long as you know its limitations. Sometimes it is helpful to think about the periodic properties as if they were describing a simple electrostatic system as long as you don't forget that quantum electrodynamics provides a fuller picture.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |