The electronegativity of an element is an incredibly helpful thumbnail signifier of its chemical 'personality', second only in importance to outer shell electron configuration. The greater the electronegativity of an element, the stronger its pull on the electrons it shares with other atoms in chemical bonds. By the time you walk into the MCAT, you really do want to have taught yourself a habit of being automatically conscious of the electronegativies of the atoms at play in any chemical situation. Such a habit makes chemistry more coherent and intuitive. Electronegativity has so many aspects of importance that it is impossible to create an exhaustive list.
One very significant role for electronegativity is in determining the polarity of covalent bonds, which then determines the degree of intermolecular force. The greater the electronegativity difference between the bonded atoms, the more polar the bond because the shared electrons spend more time around the more electronegative atom, giving it a partial negative charge and leaving the other atom partially positive. The bonded atoms form a permanent dipole. Furthermore, because there are discrete positive and negative charge densities, the dipole can be attracted by other dipoles leading to strong intermolecular forces.
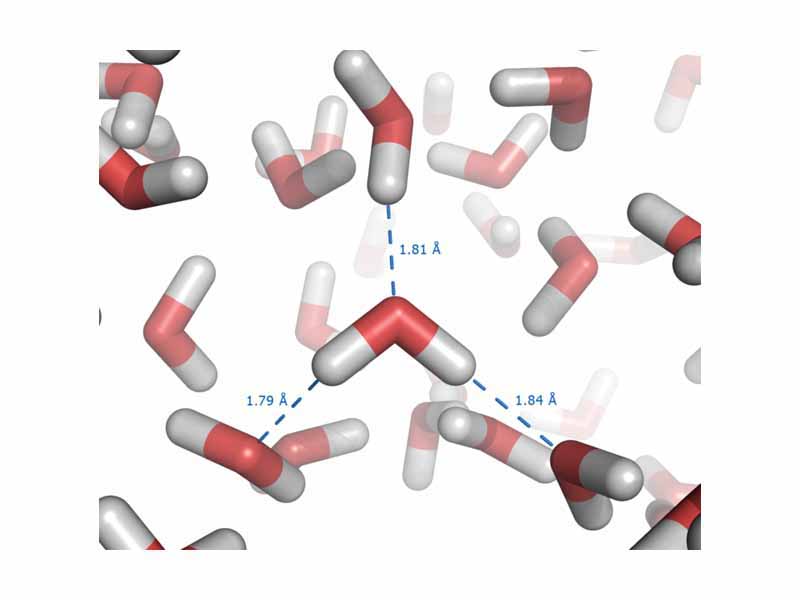
This is especially true in the case of one subtype of polar bonds, which are strong enough to earn their own name, the hydrogen bond. Hydrogen bonds are intermolecular forces that arise in substances where hydrogen is bound to a polar element, such as oxygen, and the positive pole is a bare hydrogen nucleus. Especially strong interactions result.
One very significant role for electronegativity is in determining the polarity of covalent bonds, which then determines the degree of intermolecular force. The greater the electronegativity difference between the bonded atoms, the more polar the bond because the shared electrons spend more time around the more electronegative atom, giving it a partial negative charge and leaving the other atom partially positive. The bonded atoms form a permanent dipole. Furthermore, because there are discrete positive and negative charge densities, the dipole can be attracted by other dipoles leading to strong intermolecular forces.
This is especially true in the case of one subtype of polar bonds, which are strong enough to earn their own name, the hydrogen bond. Hydrogen bonds are intermolecular forces that arise in substances where hydrogen is bound to a polar element, such as oxygen, and the positive pole is a bare hydrogen nucleus. Especially strong interactions result.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |