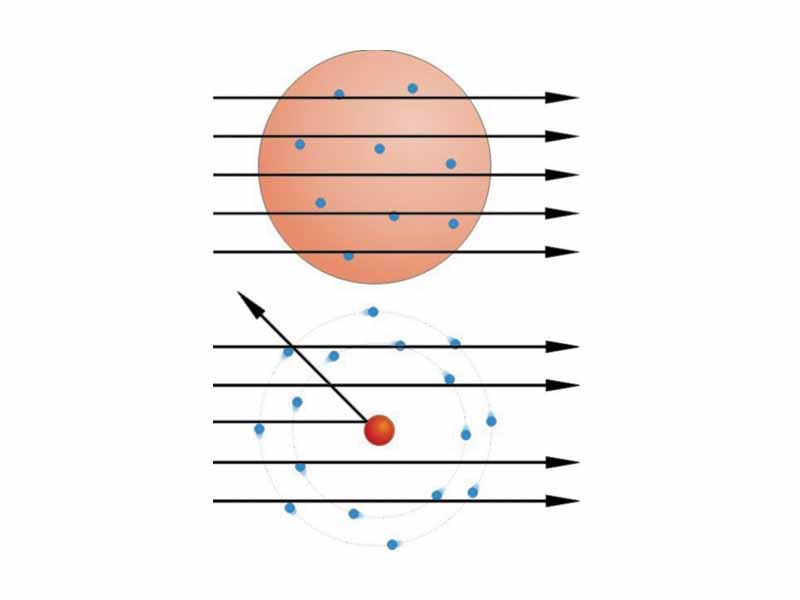
Rutherford came to the conclusion that most of the mass of the atom was concentrated in a small nucleus following the results of his gold foil experiment. In this experiment, Rutherford bombarded gold foil with alpha rays, which are streams of alpha particles. Alpha particles consist of two protons and two neutrons ejected at high speeds in alpha decay. Alpha decay is an important type of radioactive decay. Rutherford found that while almost all of the alpha particles passed straight through the foil, about 1 in 8000 were deflected. He concluded that atoms consist mostly of 'empty space'.
Prior to Rutherford's gold foil experiment, Thompson had suggested a model of the atom as a volume of positive charge with electrons spread throughout the volume. The deflections of alpha particles observed by Rutherford contradicted this model. Rutherford postulated that the positive charge was concentrated in a small region, which Rutherford called the nucleus. Rutherford developed a model similar to our solar system with electrons in orbit around the nucleus. There are problems with this model though, in that orbiting electrons, undergoing centripetal acceleration, would necessarily radiate electromagnetic waves. The electron orbit should decay and spiral into the nucleus. Secondly, the Rutherford model cannot account for the fact that the emission spectrum of a hydrogen atom is a line spectrum, an observation which serves as the basis for the subsequent Bohr model of hydrogen.
Prior to Rutherford's gold foil experiment, Thompson had suggested a model of the atom as a volume of positive charge with electrons spread throughout the volume. The deflections of alpha particles observed by Rutherford contradicted this model. Rutherford postulated that the positive charge was concentrated in a small region, which Rutherford called the nucleus. Rutherford developed a model similar to our solar system with electrons in orbit around the nucleus. There are problems with this model though, in that orbiting electrons, undergoing centripetal acceleration, would necessarily radiate electromagnetic waves. The electron orbit should decay and spiral into the nucleus. Secondly, the Rutherford model cannot account for the fact that the emission spectrum of a hydrogen atom is a line spectrum, an observation which serves as the basis for the subsequent Bohr model of hydrogen.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |