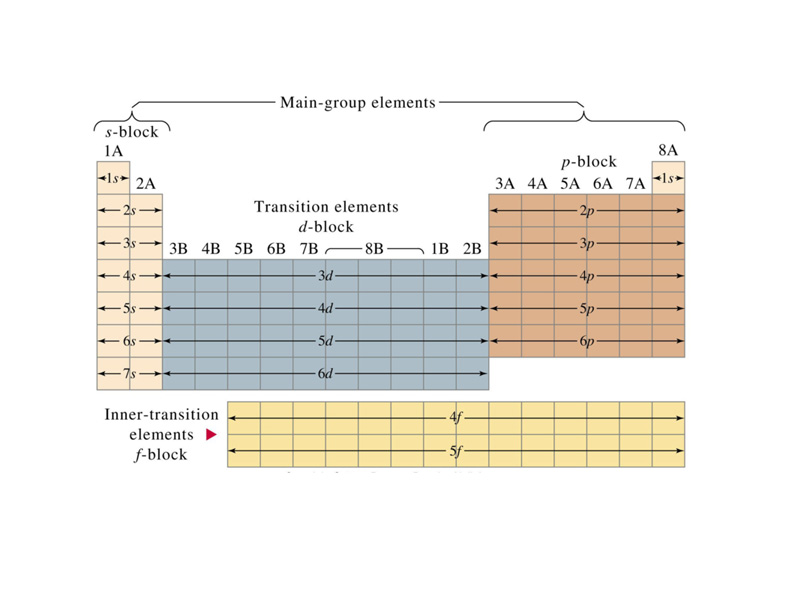
The structure of the periodic table reflects electron configuration.
The design of the periodic table reflects the observation that many properties of the chemical elements are periodic functions of their atomic number. Along with an atom's valence shell configuration, the periodic properties of an element provide a thumbnail picture of its chemical behavior. The valence plus the atomic radius, ionization energy, electron affinity and electronegativity constitute the 'personality' of an element.
Only rarely to questions appear on the MCAT that ask you directly about periodic properties, but like so many of these early physical science chapters, the material is fundamental to much else which comes later. Of the periodic properties, the electronegativity is especially crucial. Electronegativity difference between bonded atoms determines bond polarity, for example. Understanding bond polarity is necessary for predicting intermolecular forces, solubility relationships or assigning oxidation numbers.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |