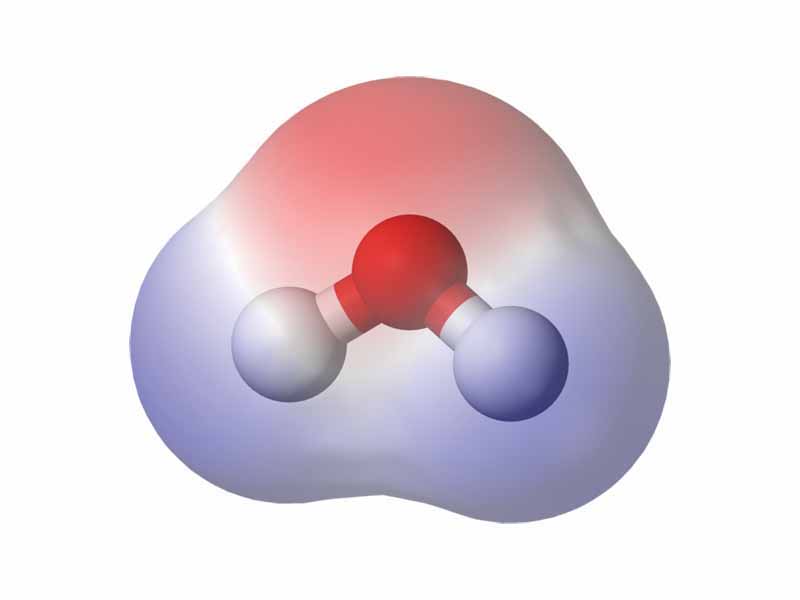
Water is a polar compound. The electrons of water's hydrogen atoms are strongly attracted to the oxygen atom, and are actually closer to oxygen's nucleus than to the hydrogen nuclei; thus, water has a relatively strong negative charge in the middle (red shade), and a positive charge at the ends (blue shade).
Electrostatic force may exist between such molecules. In near proximity, the positive pole of one molecule will be attracted to the negative pole of another.
This type of force interaction between molecules is one type of intermolecular force. Dipole-dipole, hydrogen bonding and London dispersion forces are the three types of intermolecular forces. Intermolecular force is responsible for many of the physical and solubility properties of substances.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |