When you view a chemical change, always take a moment to see the change in the light of the electrostatic force interactions of the particles involved. Step back and look at it with the lens of physics. The electrostatic force is the central force in chemistry. Take a moment to conceptualize the internal energy change in terms of the re-arrangement of electric charges, electrons and protons, through the disaggregration from reagents and reaggregation as products.
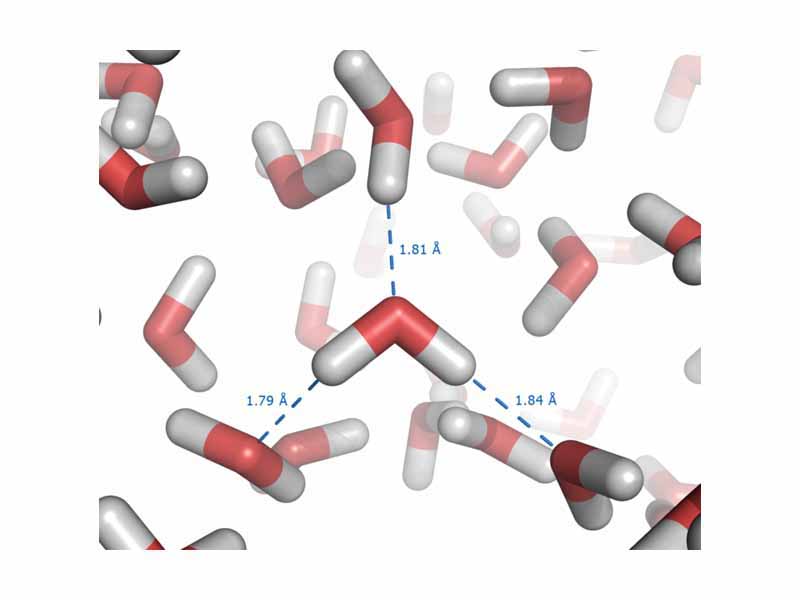
Atoms are held together by the electrostatic force between the nucleus and electrons. Two atoms in a covalent bond are held together by the electrostatic force between the nuclei of the atoms and the shared electrons in the inter nuclear space. Molecules attract one another by the electrostatic force between induced dipoles (Van der Waals attraction), permanent dipoles (dipole-dipole attraction), or hydrogen bonding, where the positive end of the dipole is a hydrogen atom).
Intermolecular forces or metallic bonding derive from electrostatic force. Electrostatic force holds the particles of a substance together in a condensed phase of matter (solid or liquid).
In a liquid solution, electrostatic forces hold together the solvent and solute. In biochemistry, the structure of a large macromolecule such as a protein, is determined by a complex interplay of electrostatic forces between the functional groups of the molecule, within the molecule itself, with associated molecules, and with the aqueous solvent. Everything you are learning about electricity in physics is preparing you to understand chemistry.
Atoms are held together by the electrostatic force between the nucleus and electrons. Two atoms in a covalent bond are held together by the electrostatic force between the nuclei of the atoms and the shared electrons in the inter nuclear space. Molecules attract one another by the electrostatic force between induced dipoles (Van der Waals attraction), permanent dipoles (dipole-dipole attraction), or hydrogen bonding, where the positive end of the dipole is a hydrogen atom).
Intermolecular forces or metallic bonding derive from electrostatic force. Electrostatic force holds the particles of a substance together in a condensed phase of matter (solid or liquid).
In a liquid solution, electrostatic forces hold together the solvent and solute. In biochemistry, the structure of a large macromolecule such as a protein, is determined by a complex interplay of electrostatic forces between the functional groups of the molecule, within the molecule itself, with associated molecules, and with the aqueous solvent. Everything you are learning about electricity in physics is preparing you to understand chemistry.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |