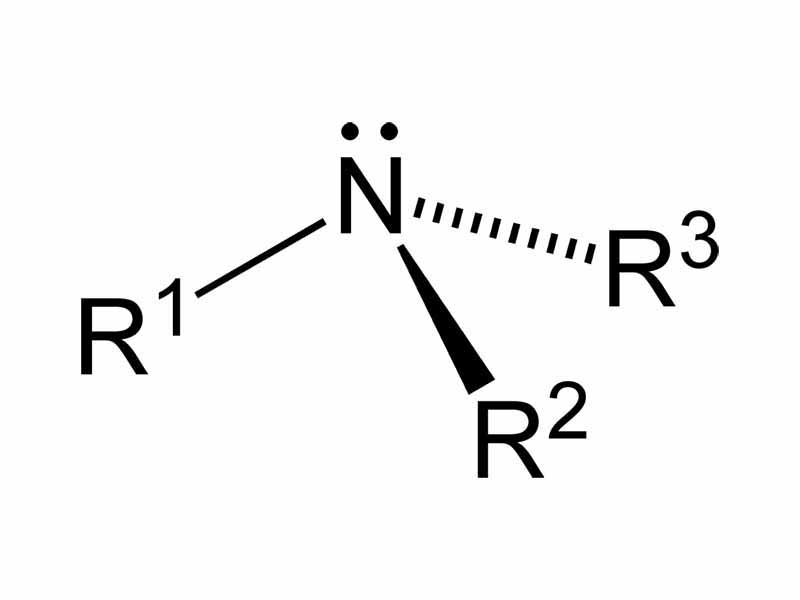
In the topic of Chemical Bonding, we discussed VSEPR (Valence Shell Electron Pair Repulsion Theory), which, based on the number of regions of high electron density around a central atom can be used to predict structures of certain molecules or ions. VSEPR questions on the MCAT are relatively common, especially those involving geometries in which nonbonded pairs of electrons are occupying positions in the geometry. There is a tendency for less-informed students to forget the nonbonded pairs and assign an incorrect geometry. You should be prepared for VSEPR questions involving amines. Amines possess a structure based on the tetrahedron with one position occupied by a nonbonding pair, so the geometry of substituents around nitrogen in an amine is pyramidal.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |