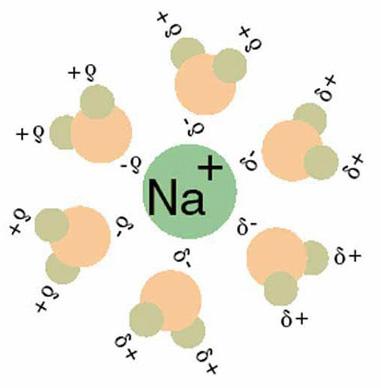
We discussed earlier how the general idea of capacitance was a useful conceptual tool for interpreting the energy of charge distributions at the molecular level. In fact, dielectrics behave during chemical processes in the same way that they behave between capacitor plates.
Similarly to how the dielectric between the plates of a capacitor reduces the voltage between the plates, the dielectric effect of water decreases the work required to separate the anions and cations of an electrolyte solute as it dissolves.
Because the field between them is weaker with the interposition of a dielectric solvent, such as water, the separation of anions and cations of a dissolving electrolyte now occur at lower energy. The aqueous solvent weakens the electric field between the separating charges.
Similarly to how the dielectric between the plates of a capacitor reduces the voltage between the plates, the dielectric effect of water decreases the work required to separate the anions and cations of an electrolyte solute as it dissolves.
Because the field between them is weaker with the interposition of a dielectric solvent, such as water, the separation of anions and cations of a dissolving electrolyte now occur at lower energy. The aqueous solvent weakens the electric field between the separating charges.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |