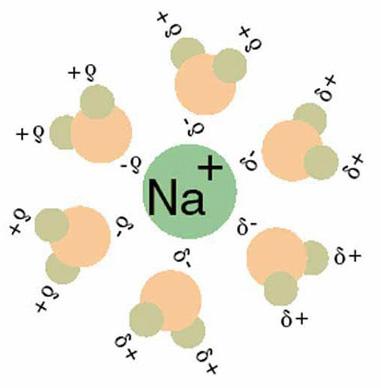
A solution is a homogeneous mixture of two or more substances, distinguished from non-homogeneous mixtures such as colloids and suspensions. There may be solid, liquid, or gaseous solutions. For certain types of liquid solutions, it is useful to describe the solution as comprised of one or more solutes dissolved within a solvent. The solubility of a solute describes its ability to dissolve in a certain solvent. A variety of concentration expressions are useful within different contexts to describe the composition of a solution including mole fraction, percent by weight, molarity, and molality. The colligative properties refer to the changes that occur in the physical properties of a solvent when a solute is added to it such as boiling point elevation, freezing point depression, and vapor pressure lowering.
For certain types of aqueous solutions of sparingly soluble electrolytes, an equilibrium will be established at low concentration between the dissolved ions and undissolved solute or precipitate which can be quantatively described using the solubility product corresponding to particular ion pairs.
If you do not know this material backwards and fowards, you will suffer on the MCAT. The topic of Solutions is one of the most important for the MCAT. Nearly every exam will require you predict solubility. You can also expect to apply what you understand about the colligative properties and puzzle through something involving heterogeneous solution equilibria. Do not neglect this material. Solutions will be on the test in a direct way.
WikiPremed Resources
Solution Chemistry Practice Items
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Solutions
In chemistry, a mixture is a substance made by combining two or more different materials in such a way that no chemical reaction occurs.
A solution is a homogeneous mixture composed of two or more substances.
Precipitation is the formation of a solid in a solution during a chemical reaction.
Concentration is the measure of how much of a given substance there is mixed with another substance.
Solubility is a physical property referring to the ability for a given substance, the solute, to dissolve in a solvent.
A aqueous solution is a solution in which the solvent is water.
Crystallization is the natural or artificial process of formation of solid crystals from a uniform solution.
Boiling-point elevation is a colligative property that states that a solution will have a higher boiling point than that of a pure solvent after the addition of a dissolved solute.
Solvation or dissolution is the process of attraction and association of molecules of a solvent with molecules or ions of a solute.
Solubility equilibrium is any chemical equilibrium between solid and dissolved states of a compound at saturation.
Colligative properties are properties of solutions that depend on the number of particles in a given volume of solvent and not on the mass of the particles.
Freezing-point depression is the difference between the freezing points of a pure solvent and a solution mixed with a solute.
A molar solution is one that contains one mole of solute per liter.
The common-ion effect is a term used to describe the effect on a solution of two dissolved solutes that contain the same ion.
Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture.
Carbonation occurs when carbon dioxide is dissolved in water or an aqueous solution.
Carbonated water is plain water into which carbon dioxide gas has been dissolved.
Dissociation is a general process in which ionic compounds separate or split into smaller molecules, ions, or radicals, usually in a reversible manner.
The mole fraction of a component in a mixture is the relative proportion of molecules belonging to the component to those in the mixture, by number of molecules.
An electrolyte is a substance containing free ions that behaves as an electrically conductive medium.
Raoult's law states that the vapor pressure of an ideal solution is dependent on the vapor pressure of each chemical component and the mole fraction of the component present in the solution.
Henry's law states that at a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
A colloid, emulsion or dispersion is a type of heterogeneous mixture consisting of a dispersed phase made of tiny particles or droplets distributed evenly throughout a continuous phase.
The term supersaturation refers to a solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances.
Percentage solution is a form of concentration expression often preferred to molarity within the biological sciences in which a 1 percent solution would have 1 g of solute dissolved in a final volume of 100 ml of solution.
An emulsion, dispersion or colloid is a mixture of two immiscible substances in which a dispersed phase made of tiny particles or droplets is distributed evenly throughout a continuous phase.
Solvation shell is a shell of any chemical species acting as a solvent, surrounding a solute species.
A suspension is a heterogenous fluid containing solid particles that are sufficiently large for sedimentation.
A partition or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium.
The van't Hoff factor is the number of moles of solute actually in solution per mole of solid solute added.
The enthalpy change of solution is the enthalpy change when one mole of a substance is dissolved completely in a large volume of a solvent at constant pressure.
An alloy is a homogeneous hybrid of two or more elements, at least one of which is a metal, and where the resulting material has metallic properties.
An azeotrope is a mixture of two or more pure compounds in such a ratio that its composition cannot be changed by simple distillation.
A multiphasic liquid is a mixture consisting of more than two immiscible liquid phases.
An ideal solution or ideal mixture is a solution in which the enthalpy of solution is zero.
Surfactants, also known as tensides, are wetting agents that lower the surface tension of a liquid and lower the interfacial tension between two liquids.
A regular solution is a solution that diverges from the behavior of an ideal solution only moderately.
A Margules function is a function added to the Raoult's law description of a liquid solution to account for deviations from ideality.
The cryoscopic constant allows one to relate molality to freezing point depression.
The ebullioscopic constant allows one to relate molality to boiling point elevation.
The Tyndall effect is the effect of light scattering on particles in colloid systems, such as suspensions or emulsions.
An eutectic mixture is a mixture at such proportions that the melting point is as low as possible, and that furthermore all the constituents crystallize simultaneously at this temperature from molten liquid solution.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |