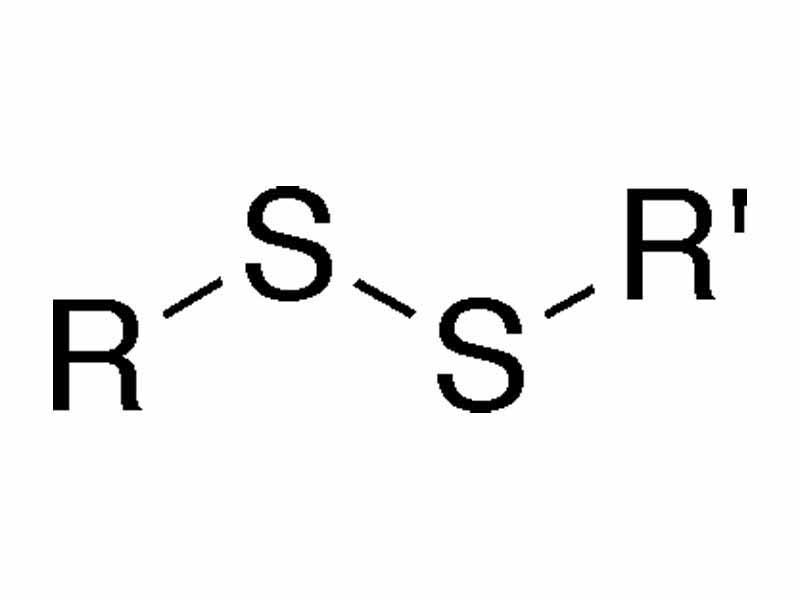Like the chemistry of organic phosphorus compounds, the chemistry of organic sulfur compounds doesn't get as much emphasis as it should in the traditional introductory organic chemistry curriculum given how many people take organic chemistry with the intention of pursuing a medical or biological sciences education. Organic chemistry taught with reference to biochemistry, reflecting the organic chemistry focus of the MCAT, would make sure students especially understand the chemistry of thioesters as well as oxidation-reduction reactions involving formation of disulfides from sulfhydrals.
WikiPremed Resources
Reaction of Alcohols with Thionyl Chloride
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Organic Sulfur Compounds
Thiols are the sulfur analogues of an alcohols.
A disulfide bond is a single covalent bond derived from the coupling of thiol groups.
Cysteine, one of the standard amino acids, possesses a thiol side chain. Along with methionine, it is one of the two standard amino acids containing sulfur.
A disulfide refers to the structural unit composed of a linked pair of sulfur atoms
One of the standard amino acids, methionine possesses a thioether side chain. Along with cysteine, it is one of the two standard amino acids containing sulfur.
The term sulfide refers to several types of chemical compounds containing sulfur in its lowest oxidation number of -2.
Thioesters are compounds resulting from the bonding of sulfur with an acyl group formed through esterification of a carboxylic acid and a thiol.
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms.
The important aprotic solvent dimethyl sulfoxide (DMSO) consists of a sulfinyl group attached to two methyl groups.
An important building block in organosulfur chemistry, carbon disulfide consists of a single carbon with double bonds to two sulfur atoms.
A thioether is a functional group in organic chemistry which is similar to ethers but with sulfur in the place of oxygen.
Thioacetals are the sulfur analogue of acetals.
Organic compounds containing the functional group SCN are called thiocyanates.
The sulfonamide functional group is a sulfone group connected to an amine group.
Carbonyl sulfide can be considered to be a hybrid of carbon dioxide and carbon disulfide, having a single carbon double bonded to an oxygen atom on one side and a sulfur atom on the other, c
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an alpha-acyloxy-thioether in the presence of acetic anhydride.
The Johnson-Corey-Chaykovsky reaction is a chemical reaction in which a carbonyl is converted to an epoxide by the action of a sulfonium ylide.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns. Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |