Electrochemistry involves chemical processes in which the two sides of an oxidation-reduction reaction are separated in space. It is the study of chemical processes which electrons are caused to move. In an electrochemical cell, charges flow from the location of oxidation (anode) to the location of reduction (cathode). Electrochemistry is an important area of applied science. Galvanic and electrolytic cells are ubiquitous in the life science laboratory. Electrochemistry also provides a framework for describing a range of phenomena which occur in living systems the most prominent example being the electron transport system of mitochondria.
WikiPremed Resources
Redox Practice Items
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Electrochemistry
Electrochemistry is a branch of chemistry that studies the reactions which take place at the interface of an electronic conductor and an ionic conductor.
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit.
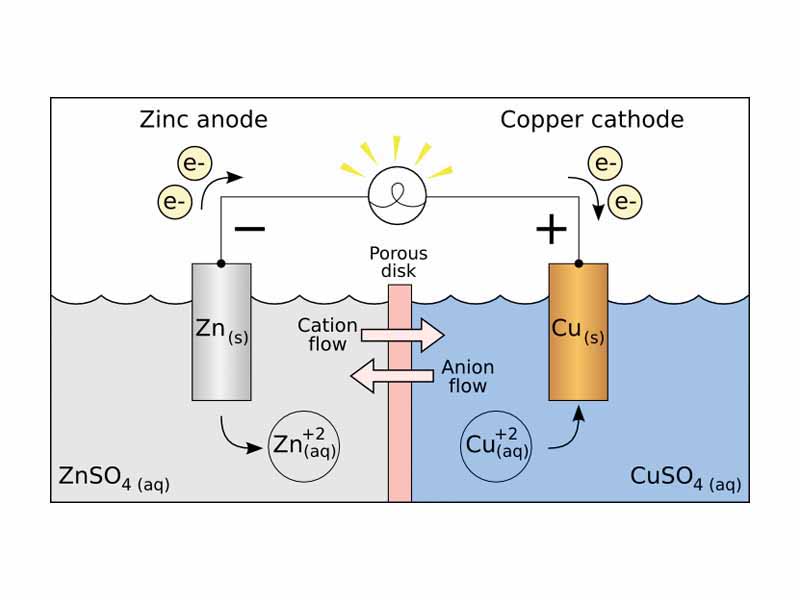
A galvanic cell (or voltaic cell) consists of two different metals connected by a salt bridge or a porous disk between the individual half-cells.
Electrolytic cells are composed of a vessel used to perform electrolysis and a cathode and anode.
An electrochemical cell is a device used for creating an electromotive force and current from chemical reactions.
An electrolyte is a substance containing free ions that behaves as an electrically conductive medium.
Electrolysis is a method of separating chemically bonded elements and compounds by passing an electric current through them.
Electromotive force or potential of a body is the work done in joules to bring a unit electric charge from infinity to the body.
An anode is an electrode through which the positive direction of electric current flows into a polarized electrical device.
A cathode is an electrode through which the positive direction of electric current flows out of a polarized electrical device.
A salt bridge is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell.
The standard electrode potential is the measure of individual voltage of any electrode at standard ambient conditions, which is at a temperature of 298K, solutes at a concentration of 1 M, and gases at a pressure of 1 bar.
The Nernst equation gives the electrode potential relative to the standard electrode potential of the electrode couple as a function of component concentrations.
The standard hydrogen electrode is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials.
The Faraday constant is the amount of electric charge in one mole of electrons.
Electroplating is the process of using electrical current to coat an electrically conductive object with a relatively thin layer of metal.
A half cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally-occurring Helmholtz double layer.
The Palladium-Hydrogen electrode is one of the common reference electrodes used in electrochemical study.
A reference electrode is an electrode which has a stable and well-known electrode potential.
A concentration cell is an electrochemical cell that has two equivalent half-cells of the same material differing only in molarity.
A primary cell is any kind of electrochemical cell in which the electrochemical reaction of interest is not reversible.
A rechargeable battery, also known as a storage battery, is a group of two or more secondary cells.
The voltaic pile is the first modern electric battery, invented by Alessandro Volta in 1800.
Electroanalytical chemistry involves the analysis of chemical species through the use of electrochemical methods.
The galvanic series determines the nobility of metals and semi-metals by submerging two metals in an electrolyte, while electrically connected, and determining the less noble as the one that experiences corrosion.
Anodizing is an electrolytic passivation process used to increase the thickness and density of the natural oxide layer on the surface of metal parts.
Lead-acid batteries are the oldest type of rechargeable battery.
Electrowinning, also called electroextraction, is the electrodeposition of metals from their ores that have been put in solution or liquefied.
Cyclic voltammetry is a type of potentiodynamic electrochemical measurement in which a voltage is applied to a working electrode in solution and current flowing at the working electrode is plotted versus the applied voltage.
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes in which information about an analyte is obtained by measuring the current as the potential is varied.
Measuring a quantity of electricity by mass change of the electrodes, the copper coulometer consists of two identical copper electrodes immersed into the slightly acidic pH-buffered solution of copper sulfate.
A redox electrode is an electrode made from electron-conductive material and characterized by high chemical stability in the solution under test.
The Dry-Pile (also known as the Duluc pile or Zamboni pile) is a high voltage low current semi-permanent electric battery developed in the early 1800s and constructed from silver foil, zinc foil, and paper.
The saturated calomel electrode is a reference electrode based on the reaction between elemental mercury and mercury chloride.
A silver chloride electrode is a type of reference electrode, used for measuring electrochemical potential, which is the most commonly used reference electrode for testing cathodic protection corrosion control systems in sea water environments.
Cathodic protection is a technique to control the corrosion of a metal surface by making that surface the cathode of an electrochemical cell.
Differential pulse voltammetry is a kind of electrochemical measurement which can be considered as a series of regular voltage pulses superimposed on a linearly changing voltage, in which the resulting current is measured between the ramped baseline voltage and the pulse voltage.
Chronoamperometry is an electrochemical technique in which the potential of the working electrode is stepped, and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time.
The Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry.
The Betts electrolytic process is an industrial process for separating lead and bismuth.
The Castner process is a process for manufacturing sodium metal by electrolysis of molten sodium hydroxide.
The Castner-Kellner process is a method of electrolysis on an aqueous alkali chloride solution to produce the corresponding alkali hydroxide.
A chloralkali process is any electrolytic process which produces chlorine or a related oxidizer, such as bleaching powder, and an alkaline salt such as sodium hydroxide or sodium carbonate.
 The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns.ô ô Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT. The WikiPremed MCAT Course is a comprehensive course in the undergraduate level general sciences. Undergraduate level physics, chemistry, organic chemistry and biology are presented by this course as a unified whole within a spiraling curriculum. Please read our policies on Privacy and Shipping & Returns.ô ô Contact Us. MCAT is a registered trademark of the Association of American Medical Colleges, which does not endorse the WikiPremed Course. WikiPremed offers the customers of our publications or our teaching services no guarantees regarding eventual performance on the MCAT.

WikiPremed is a trademark of Wisebridge Learning Systems LLC. The work of WikiPremed is published under a Creative Commons Attribution NonCommercial ShareAlike License. There are elements of work here, such as a subset of the images in the archive from WikiPedia, that originated as GNU General Public License works, so take care to follow the unique stipulations of that license in printed reproductions. |